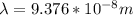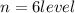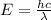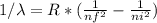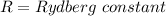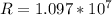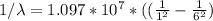Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3
You know the right answer?
Calculate the shortest wavelength of the electromagnetic radiation emitted by the hydrogen atom in u...
Questions


Social Studies, 09.10.2021 14:00


Social Studies, 09.10.2021 14:00

Mathematics, 09.10.2021 14:00


Mathematics, 09.10.2021 14:00


History, 09.10.2021 14:00

Mathematics, 09.10.2021 14:00


Mathematics, 09.10.2021 14:00


Mathematics, 09.10.2021 14:00


Mathematics, 09.10.2021 14:00



Social Studies, 09.10.2021 14:00