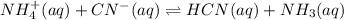Identify the bronsted-lowry acid and the bronsted-lowry base in this reaction on the left side of each of the following equations, and also identify the conjugate acid and conjugate base of each on the right side. mathrm { NH } _ { 4 } ^ { + } ( a q ) + mathrm { CN } ^ { - } ( a q ) rightleftharpoons mathrm { HCN } ( a q ) + mathrm { NH } _ { 3 } ( a q )

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1


Chemistry, 23.06.2019 01:00
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer.
Answers: 1
You know the right answer?
Identify the bronsted-lowry acid and the bronsted-lowry base in this reaction on the left side of ea...
Questions


Mathematics, 05.11.2020 21:20


Mathematics, 05.11.2020 21:20


Mathematics, 05.11.2020 21:20


History, 05.11.2020 21:20


Mathematics, 05.11.2020 21:20


Mathematics, 05.11.2020 21:20


Biology, 05.11.2020 21:20


Mathematics, 05.11.2020 21:20


English, 05.11.2020 21:20

Social Studies, 05.11.2020 21:20

Chemistry, 05.11.2020 21:20

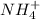 is an acid,
is an acid, 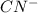 is a base,
is a base, 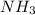 is conjugate base and
is conjugate base and 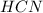 is conjugate acid
is conjugate acid