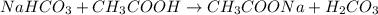Chemistry, 21.06.2021 17:50 danielcano12281621
PLEASE HELP
The average number of drops of NaHCO3 in the titration lab was 142. Calculate the number of moles of NaCHO3 that were required to neutralize the CH3COOH in the vinegar.
- 1 mole of acetic acid was required for 2 moles of sodium bicarbonate
- 1 mole of acetic acid was required for 1 mole of sodium bicarbonate
- 2 mole of acetic acid was required for 1 mole of sodium bicarbonate
- 1 mole of acetic acid was required for 3 mole of sodium bicarbonate

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 23.06.2019 00:20
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3


Chemistry, 23.06.2019 10:00
Compare and contrast an assemblage and a pollen fingerprint by defying both and giving examples of each from the chapter.
Answers: 3
You know the right answer?
PLEASE HELP
The average number of drops of NaHCO3 in the titration lab was 142. Calculate the numbe...
Questions

Chemistry, 11.07.2021 14:00

Arts, 11.07.2021 14:00

English, 11.07.2021 14:00

World Languages, 11.07.2021 14:00

Mathematics, 11.07.2021 14:00

English, 11.07.2021 14:00


Social Studies, 11.07.2021 14:00

World Languages, 11.07.2021 14:00

Mathematics, 11.07.2021 14:00


Mathematics, 11.07.2021 14:00

English, 11.07.2021 14:00

Mathematics, 11.07.2021 14:00

Mathematics, 11.07.2021 14:00

Mathematics, 11.07.2021 14:00



Mathematics, 11.07.2021 14:00