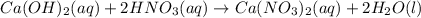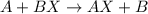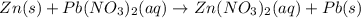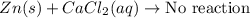Chemistry, 22.06.2021 06:10 chenepiernas
Part 1: Name the type of chemical reaction that occurs when calcium hydroxide (Ca(OH)2) reacts with nitric acid (HNO3). Part 2: Explain why zinc (Zn) would react with lead nitrate (Pb(NO3)2) but not with calcium chloride (CaCl2).

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 23.06.2019 04:50
The diagin dilutepage 6 of 12a6a5(a)fluorine, chlorine, bromine and iodine are placed in the same group of theperiodic table.state the common name used to describe elements in this group.(i)state the group in which the elements are placed and explain whythey are placed in that group.(ii)which of the above named elements is a solid at roomtemperature and pressure?
Answers: 2

Chemistry, 23.06.2019 05:40
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1
You know the right answer?
Part 1: Name the type of chemical reaction that occurs when calcium hydroxide (Ca(OH)2) reacts with...
Questions


Mathematics, 06.07.2019 16:00

Mathematics, 06.07.2019 16:00

Social Studies, 06.07.2019 16:00


Business, 06.07.2019 16:00

History, 06.07.2019 16:00

Mathematics, 06.07.2019 16:00

History, 06.07.2019 16:00






Mathematics, 06.07.2019 16:00

Arts, 06.07.2019 16:00

History, 06.07.2019 16:00


Geography, 06.07.2019 16:00

History, 06.07.2019 16:00