
Chemistry, 22.06.2021 14:50 lebron06james
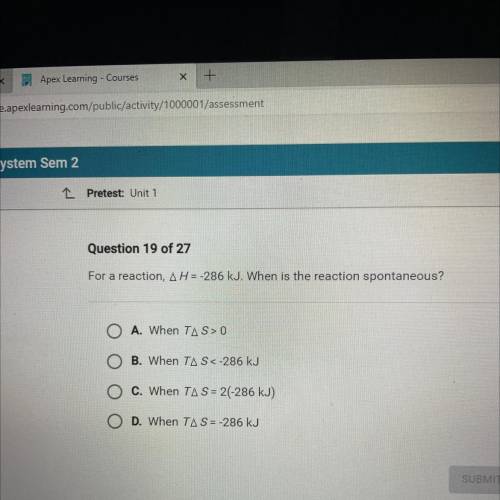
For a reaction, AH = -286 kJ. When is the reaction spontaneous?
O A. When TAS> 0
O B. When TAS<-286 kJ
O C. When TA S = 2(-286 kJ)
O D. When TAS = -286 kJ

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1


Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1
You know the right answer?
For a reaction, AH = -286 kJ. When is the reaction spontaneous?
O A. When TAS> 0
O B. When...
O B. When...
Questions

Social Studies, 02.10.2021 14:00






Biology, 02.10.2021 14:00

Mathematics, 02.10.2021 14:00

History, 02.10.2021 14:00

Social Studies, 02.10.2021 14:00

Mathematics, 02.10.2021 14:00



Mathematics, 02.10.2021 14:00



Mathematics, 02.10.2021 14:00





