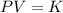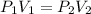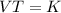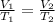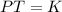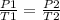Select the keyword or phrase that will best complete each sentence. law is a gas law that relates pressure and volume and states that for a fixed amount of gas at constant temperature, the pressure and volume of the gas are related. law is a gas law that states that for a fixed amount of gas at constant pressure, the volume of the gas is propotional to its Kelvin temperature. law is a law that states that the total pressure of a gas mixture is equal to the sum of the partial pressure of its component gases. law is a gas law that states that the volume of a gas is proportional to the number of moles of present when the pressure and temperature are held constant. law is a gas law that states for a fixed amount of gas at constant volume, the pressure of the gas is proportional to its Kelvin temperature. The law is a gas law that relates pressure, volume, and temperature. The law is the equation PV

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1
You know the right answer?
Select the keyword or phrase that will best complete each sentence. law is a gas law that relates pr...
Questions


English, 25.08.2019 18:00

History, 25.08.2019 18:00

History, 25.08.2019 18:00


Physics, 25.08.2019 18:00




Computers and Technology, 25.08.2019 18:00




Mathematics, 25.08.2019 18:00



Chemistry, 25.08.2019 18:00



English, 25.08.2019 18:00