
Chemistry, 22.06.2021 18:00 keiracoles
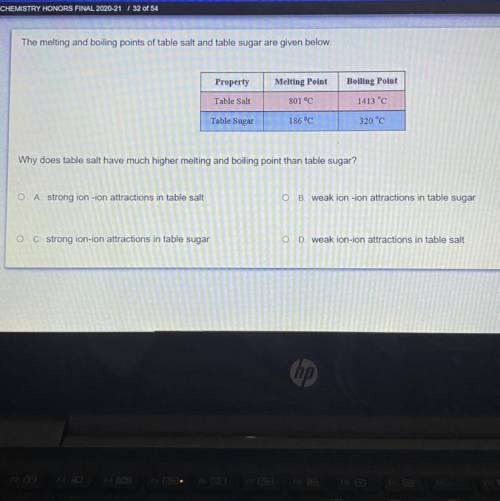
The melting and boiling points of table salt and table sugar are given below.
Why does table salt have much higher melting and boiling point than table sugar?
o A strong ion -ion attractions in table salt
O B. weak ion-ion attractions in table sugar
O C. strong ion-ion attractions in table sugar
O
D. weak ion-ion attractions in table salt

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3
You know the right answer?
The melting and boiling points of table salt and table sugar are given below.
Why does table salt h...
Questions






Mathematics, 11.06.2020 20:57




Mathematics, 11.06.2020 20:57

History, 11.06.2020 20:57

Health, 11.06.2020 20:57


Biology, 11.06.2020 20:57

Mathematics, 11.06.2020 20:57

Mathematics, 11.06.2020 20:57

Social Studies, 11.06.2020 20:57

Mathematics, 11.06.2020 20:57




