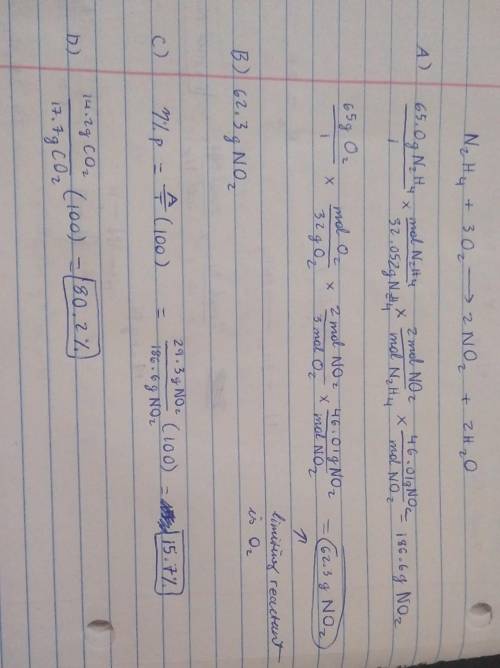19) Hydrazine, N2H4, a substance used as rocket fuel, reacts with oxygen as follows:
N2H4 (1) + 3 O2(g) → 2 NO2(g) + 2 H20 (8)
A) If 65.0 g of hydrazine are reacted with 65.0 g of oxygen, which is the limiting reactant?
usg of Oz X Imol
.
B) How many grams of NO2 are produced from the limiting reactant in part A?
C) If 29.3 g of NO2 are obtained from the reaction in Part A, what is the percent yield?
20) What is the percent yield if 14.2 g of CO2 were produced and 17.7 g were calculated to be
produced from the reaction?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is common about these molecules? a.their atoms are held together by covalent bonds. b.they are all made up of the same two atoms. c.their atoms are held together by ionic bonds. d.they are all made up of oxygen atoms only.
Answers: 3

Chemistry, 21.06.2019 20:30
There is an area in idaho named craters of the moon where most of the ground is covered with basalt, adark gray, igneous rock with no visibl crystals. what can you infer about the geographical history of the area?
Answers: 1

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1
You know the right answer?
19) Hydrazine, N2H4, a substance used as rocket fuel, reacts with oxygen as follows:
N2H4 (1) + 3 O...
Questions




Mathematics, 05.03.2021 23:00

Social Studies, 05.03.2021 23:00


Mathematics, 05.03.2021 23:00

Mathematics, 05.03.2021 23:00

Health, 05.03.2021 23:00

Mathematics, 05.03.2021 23:00



English, 05.03.2021 23:00

Biology, 05.03.2021 23:00

Mathematics, 05.03.2021 23:00

English, 05.03.2021 23:00

Mathematics, 05.03.2021 23:00