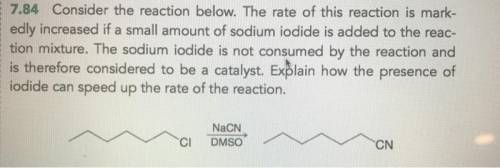
Chemistry, 23.06.2021 01:50 karennayeli
The rate of this reaction is markedly increased if a small amount of sodium iodide is added to the reaction mixture. The sodium iodide is not consumed by the reaction and is therefore considered to be a catalyst. Explain how the presence of iodide can speed up the rate of the reaction.

Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 04:00
What two categories of toxins were present in the air at dish,texas as a result of the gas pipelines that pass through the area
Answers: 1

Chemistry, 23.06.2019 06:00
Each step in the following process has a yield of 70% ch4 + 4cl2 yield ccl4 +4hcl ccl4 + 2hf yield ccl2f2 + 2hcl of 4.50 mole ch4 reacts what is the total amount of hcl produced
Answers: 3

Chemistry, 23.06.2019 13:00
If volume remains the same while the mass of a substance the density of the substance
Answers: 1
You know the right answer?
The rate of this reaction is markedly increased if a small amount of sodium iodide is added to the r...
Questions


Mathematics, 01.07.2019 23:30

Computers and Technology, 01.07.2019 23:30

Mathematics, 01.07.2019 23:30

History, 01.07.2019 23:30

Mathematics, 01.07.2019 23:30



Mathematics, 01.07.2019 23:30





English, 01.07.2019 23:30



History, 01.07.2019 23:30

History, 01.07.2019 23:30