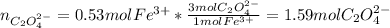Chemistry, 23.06.2021 07:40 smithmalyk4
Kc is 1.67 x 10^20 at 25 °C for the formation of iron(III) oxalate complex ion:
Fe^3+ (aq) + 3C2O4^2- (aq) <--> [Fe(C204)3]^3- (aq)
Determine the number of moles of C2O4^2- used to react with 0.53 moles of Fe^3+.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2


Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2
You know the right answer?
Kc is 1.67 x 10^20 at 25 °C for the formation of iron(III) oxalate complex ion:
Fe^3+ (aq) + 3C2O4^...
Questions


Computers and Technology, 14.04.2020 18:36










Social Studies, 14.04.2020 18:36








Mathematics, 14.04.2020 18:36

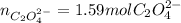
![Kc=\frac{[[Fe(C_2O_4)_3]^{3-}]}{[Fe^{3+}][C_2O_4^{2-}]^3}](/tpl/images/1382/0865/5bd65.png)