

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 23.06.2019 05:00
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 2

Chemistry, 23.06.2019 06:00
Nthis lab, you will do experiments to identify types of changes. using the question format you learned (shown above), write an investigative question that you can answer by doing these experiments
Answers: 3
You know the right answer?
Consider the following intermediate chemical equations.
CH2(g) →C(s) + 2H2(g)
CC1.(g) → C(s)...
CC1.(g) → C(s)...
Questions


Mathematics, 09.06.2021 22:20

Social Studies, 09.06.2021 22:20


Mathematics, 09.06.2021 22:20

Mathematics, 09.06.2021 22:20


History, 09.06.2021 22:20







Mathematics, 09.06.2021 22:20

Mathematics, 09.06.2021 22:20



Mathematics, 09.06.2021 22:20

English, 09.06.2021 22:20

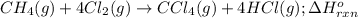
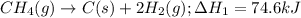
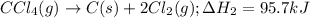
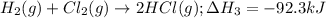
![\Delta H^o_{rxn}=[1\times (\Delta H_1)] + [1\times (-\Delta H_2)] + [2\times (\Delta H_3)]](/tpl/images/1382/1138/89421.png)
![\Delta H^o_{rxn}=[1\times (74.6)] + [1\times (-95.7)] + [2\times (-92.3)]\\\\\Delta H^o_{rxn}=-205.7kJ](/tpl/images/1382/1138/33de2.png)


