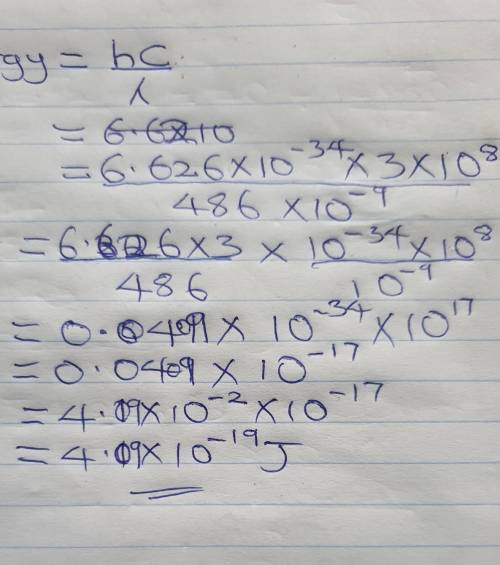The hydrogen emission spectrum is shown below. What is the energy of the
486 nm emission line? (The speed of light in a vacuum is 3.00 x 10^8 m/s, and Planck's constant is 6.626 x 10^-34 Jos.)
A. 2.44 x 10^18 J
B. 7.33 x 10^26 J
C. 6.17 x 10^14 J
D. 4.09 x 10^-19 J

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1

Chemistry, 23.06.2019 06:30
Which of the following steps is not likely to take place during cellular respiration? (5 points) select one: a. oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. c. simple sugar breaks down. d. energy is used up.
Answers: 1
You know the right answer?
The hydrogen emission spectrum is shown below. What is the energy of the
486 nm emission line? (The...
Questions

Chemistry, 22.03.2021 18:30

Biology, 22.03.2021 18:30


English, 22.03.2021 18:30

Mathematics, 22.03.2021 18:30

Social Studies, 22.03.2021 18:30


Social Studies, 22.03.2021 18:30


History, 22.03.2021 18:30


History, 22.03.2021 18:30

Computers and Technology, 22.03.2021 18:30

English, 22.03.2021 18:30

Chemistry, 22.03.2021 18:30

Mathematics, 22.03.2021 18:30

Mathematics, 22.03.2021 18:30


Mathematics, 22.03.2021 18:30

Mathematics, 22.03.2021 18:30