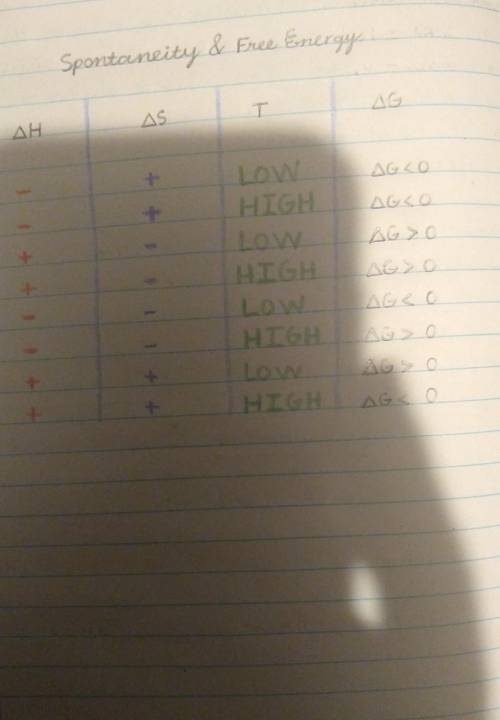
At what temperatures is a reaction that has a positive change in entropy
spontaneous?
A. At a...

Chemistry, 23.06.2021 19:20 ayoismeisalex
At what temperatures is a reaction that has a positive change in entropy
spontaneous?
A. At all temperatures for exothermic reactions
B. At lower temperatures for endothermic reactions
C. At all temperatures for endothermic reactions
D. At no temperatures for exothermic reactions

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2
You know the right answer?
Questions

History, 15.01.2020 10:31

Mathematics, 15.01.2020 10:31

English, 15.01.2020 10:31



Spanish, 15.01.2020 10:31




Social Studies, 15.01.2020 10:31




Mathematics, 15.01.2020 10:31


History, 15.01.2020 10:31


Mathematics, 15.01.2020 10:31