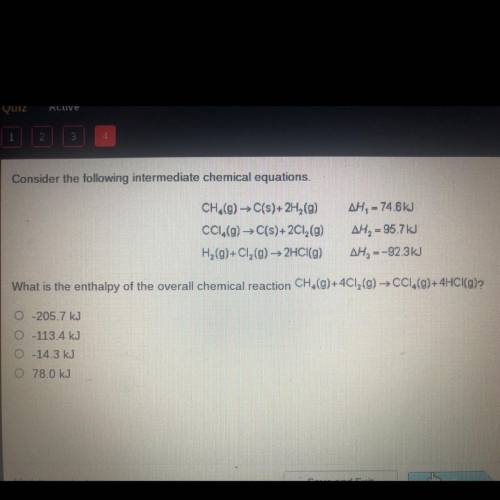
Consider the following intermediate chemical equations.
CH.(g) → C(s)+2H (9)
CC1.(g) → C(s)+2...

Chemistry, 23.06.2021 19:50 nataliajaquez02
Consider the following intermediate chemical equations.
CH.(g) → C(s)+2H (9)
CC1.(g) → C(s)+2Cl2(g)
H2(g)+C1, (g) → 2HCl(g)
AH, = 74.6 kJ
AH, = 95.7 kJ
AH, =-92.3kJ
What is the enthalpy of the overall chemical reaction CH,(g)+4C12(g) → CC1,(9)+4HCI(g)?
O-205.7 kJ
0-113.4 kJ
-14.3 kJ
0 78.0 kJ

Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 00:00
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2

Chemistry, 23.06.2019 01:00
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 03:00
A0.100-kilogram apple hangs in a tree 1.50 meter above the ground. ignore frictional effects, the total mechanical energy of the apples is
Answers: 1
You know the right answer?
Questions

Mathematics, 15.07.2019 04:00


Mathematics, 15.07.2019 04:00

Health, 15.07.2019 04:00

Mathematics, 15.07.2019 04:00




Mathematics, 15.07.2019 04:00



Mathematics, 15.07.2019 04:00


Mathematics, 15.07.2019 04:00


English, 15.07.2019 04:00

Chemistry, 15.07.2019 04:00


Mathematics, 15.07.2019 04:00

English, 15.07.2019 04:00



