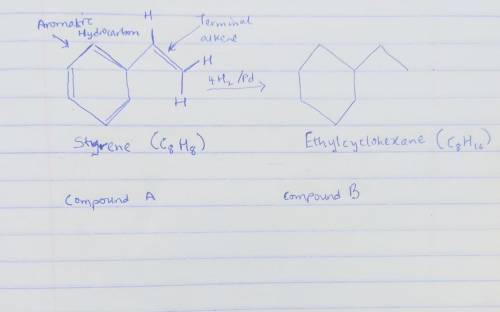
Compound A has the formula C8H8. It reacts rapidly with acidic KMnO4 but reacts with only 1 equivalent of H2 over a palladium catalyst. On hydrogenation under conditions that reduce aromatic rings, A reacts with 4 equivalents of H2, and hydrocarbon B, C8H16, is produced. The reaction of A with KMnO4 gives CO2 and a carboxylic acid C, C7H6O2.
Required:
Draw the structure of compound B below.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:00
Your answer should have the same number or significant figures as a he starting measurement. 3201 ml =
Answers: 2

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1

You know the right answer?
Compound A has the formula C8H8. It reacts rapidly with acidic KMnO4 but reacts with only 1 equivale...
Questions


Social Studies, 02.07.2019 07:30

Mathematics, 02.07.2019 07:30


Mathematics, 02.07.2019 07:30

History, 02.07.2019 07:30


Biology, 02.07.2019 07:30


History, 02.07.2019 07:30

Mathematics, 02.07.2019 07:30

Biology, 02.07.2019 07:30

Mathematics, 02.07.2019 07:30



History, 02.07.2019 07:30

Biology, 02.07.2019 07:30

Business, 02.07.2019 07:30

History, 02.07.2019 07:30

Business, 02.07.2019 07:30