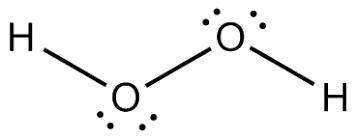
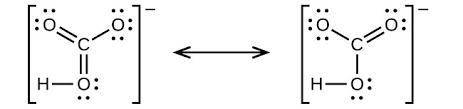
Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 23.06.2019 04:00
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2

Chemistry, 23.06.2019 10:30
Which of the following characteristics are true of enzymes? check all that apply. a.)the structure of an enzyme can change if conditions change. b.)a single enzyme can normally catalyze a wide variety of reactions under many conditions. c.)enzymes are found only in nonliving systems. d.)enzymes allow living things to regulate body conditions through feedback mechanisms. e.)enzymes bind to specific substrates in specific ways. f.)enzymes increase the rate of reaction. g.)when shown in energy-reaction diagrams, enzymes represent the higher activation energy.
Answers: 1

Chemistry, 23.06.2019 12:30
What would happen to a weak base dissociation equilibrium if more products we added
Answers: 1
You know the right answer?
Toothpastes containing sodium hydrogen carbonate (sodium bicarbonate) and hydrogen peroxide are wide...
Questions


History, 14.10.2019 16:00



Business, 14.10.2019 16:00


History, 14.10.2019 16:00


Mathematics, 14.10.2019 16:00

Physics, 14.10.2019 16:00


Mathematics, 14.10.2019 16:00






Arts, 14.10.2019 16:00

History, 14.10.2019 16:00