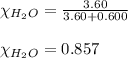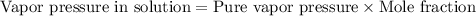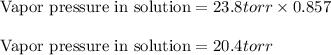Chemistry, 24.06.2021 19:50 ryliepeloquinf
If 0.600 mol of a nonvolatile nonelectrolyte are dissolved in 3.60 mol of water, what is the vapor pressure PH2O of the resulting solution? The vapor pressure of pure water is 23.8 torr at 25 ∘C . Express your answer with the appropriate units. View Available Hint(s) Hint 1. Determine the mole fraction of wateropened hint What is the mole fraction of water, XH2O, in this solution?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1
You know the right answer?
If 0.600 mol of a nonvolatile nonelectrolyte are dissolved in 3.60 mol of water, what is the vapor p...
Questions



Social Studies, 20.10.2020 06:01


Mathematics, 20.10.2020 06:01

Mathematics, 20.10.2020 06:01

Social Studies, 20.10.2020 06:01


Mathematics, 20.10.2020 06:01

Mathematics, 20.10.2020 06:01

Chemistry, 20.10.2020 06:01



Mathematics, 20.10.2020 06:01

Mathematics, 20.10.2020 06:01



Mathematics, 20.10.2020 06:01

Mathematics, 20.10.2020 06:01

Mathematics, 20.10.2020 06:01

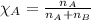 .....(1)
.....(1)