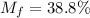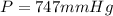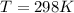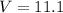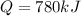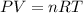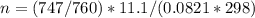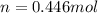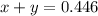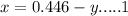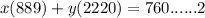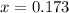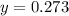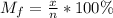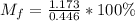Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 22.06.2019 22:30
Consider a culture medium on which only gram-positive organisms such as staphylococcus aureus colonies can grow due to an elevated nacl level. a yellow halo surrounds the growth, indicating the bacterium fermented a sugar in the medium, decreasing the ph as a result and changing the color of a ph indicator chemical. this type of medium would be referred to as a differential and enrichment culture.
Answers: 2
You know the right answer?
A gaseous fuel mixture stored at 747 mmHg and 298 K contains only methane (CH4) and propane (C3H8)....
Questions

Mathematics, 17.03.2020 23:29

English, 17.03.2020 23:29

Computers and Technology, 17.03.2020 23:29

Mathematics, 17.03.2020 23:29


Mathematics, 17.03.2020 23:29




Mathematics, 17.03.2020 23:29

History, 17.03.2020 23:29

History, 17.03.2020 23:29

History, 17.03.2020 23:29

Arts, 17.03.2020 23:29


Mathematics, 17.03.2020 23:29

Health, 17.03.2020 23:29


English, 17.03.2020 23:29