Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 16:00
Nickel crystallizes in the face-centered cubic (fcc) lattice. the density of the metal is 8902 kg/m3. calculate the radius of a nickel atom.
Answers: 1


Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1
You know the right answer?
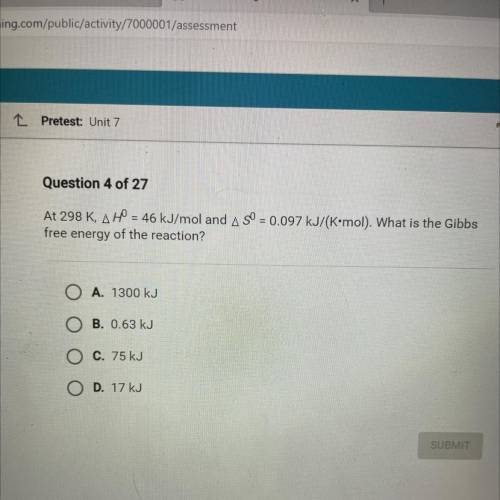
At 298 K, AH = 46 kJ/mol and A SO = 0.097 kJ/(K•mol). What is the Gibbs
free energy of the reaction...
Questions





History, 29.10.2020 17:00


Computers and Technology, 29.10.2020 17:00

Chemistry, 29.10.2020 17:00











Biology, 29.10.2020 17:00