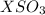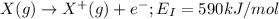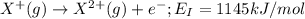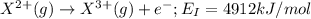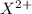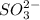Chemistry, 25.06.2021 04:50 Franklyn3834
the first three ionization energies of an element x are 590, 1145, and 4912 kj mol-1. what is the most likely formula of the compound formed between the stable ion of x and sulfite.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1


Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1
You know the right answer?
the first three ionization energies of an element x are 590, 1145, and 4912 kj mol-1. what is the mo...
Questions

Mathematics, 14.05.2021 17:20





Mathematics, 14.05.2021 17:20

Mathematics, 14.05.2021 17:20





History, 14.05.2021 17:20

Social Studies, 14.05.2021 17:20




Health, 14.05.2021 17:20


Geography, 14.05.2021 17:20

Mathematics, 14.05.2021 17:20