
Chemistry, 25.06.2021 14:00 maggie9459
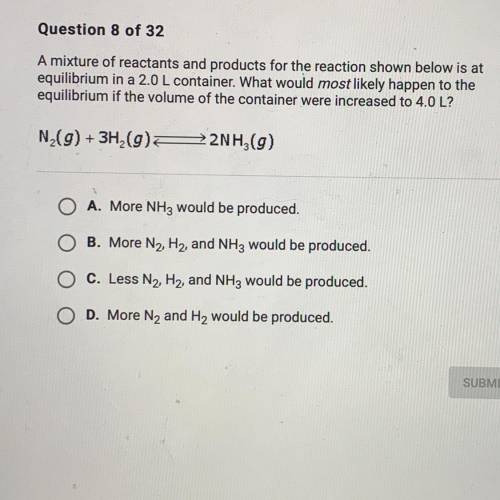
A mixture of reactants and products for the reacting shown below is at equilibrium 2.0 L container. What would most likely happen to the equilibrium if the volume of the container were increased to 4.0 L?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
You know the right answer?
A mixture of reactants and products for the reacting shown below is at equilibrium 2.0 L container....
Questions

Mathematics, 14.04.2021 07:00

History, 14.04.2021 07:00


Mathematics, 14.04.2021 07:00

Mathematics, 14.04.2021 07:00

History, 14.04.2021 07:00

Mathematics, 14.04.2021 07:00


Mathematics, 14.04.2021 07:00


English, 14.04.2021 07:00


English, 14.04.2021 07:10





Mathematics, 14.04.2021 07:10

History, 14.04.2021 07:10

Mathematics, 14.04.2021 07:10



