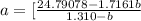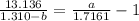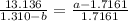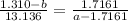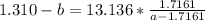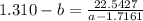Chemistry, 26.06.2021 23:20 haleylayne74
How do I solve for a and b using the Van Der Waals equation using only the given values: P= 1 atm, V= 1.310 L, and T= 160 K

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:30
1. baking powder is a 1: 1 molar mixture of cream of tartar (khc4h4o6) and baking soda (nahco3). a recipe calls for two teaspoons (a total of 8.0 grams) of cream of tartar. how much baking soda must be added for both materials to react completely?
Answers: 2

Chemistry, 21.06.2019 18:30
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2

Chemistry, 21.06.2019 23:00
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

You know the right answer?
How do I solve for a and b using the Van Der Waals equation using only the given values: P= 1 atm, V...
Questions

Mathematics, 05.07.2019 08:00

Mathematics, 05.07.2019 08:00



Mathematics, 05.07.2019 08:00



Mathematics, 05.07.2019 08:00

Biology, 05.07.2019 08:00

Mathematics, 05.07.2019 08:00


English, 05.07.2019 08:00


Mathematics, 05.07.2019 08:00

English, 05.07.2019 08:00

Mathematics, 05.07.2019 08:00

History, 05.07.2019 08:00

Mathematics, 05.07.2019 08:00

Mathematics, 05.07.2019 08:00

Mathematics, 05.07.2019 08:00

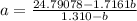
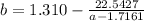
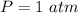
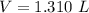
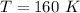
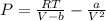
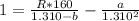
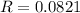
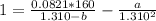
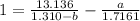 ----- (a)
----- (a)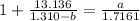
![a = [1 + \frac{13.136}{1.310 - b}] * 1.7161](/tpl/images/1384/6026/5f4fa.png)
![a = [\frac{1.310 - b+13.136}{1.310 - b}] * 1.7161](/tpl/images/1384/6026/cb054.png)
![a = [\frac{14.446- b}{1.310 - b}] * 1.7161](/tpl/images/1384/6026/4a827.png)