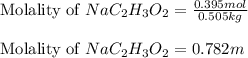Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

You know the right answer?
An aqueous sodium acetate, NaC2H3O2 , solution is made by dissolving 0.395 mol NaC2H3O2 in 0.505 kg...
Questions

Biology, 19.05.2020 14:02

Mathematics, 19.05.2020 14:02

Biology, 19.05.2020 14:02




Biology, 19.05.2020 14:02




English, 19.05.2020 14:02

Mathematics, 19.05.2020 14:02

Mathematics, 19.05.2020 14:02

Mathematics, 19.05.2020 14:02


Mathematics, 19.05.2020 14:02


Mathematics, 19.05.2020 14:02


Computers and Technology, 19.05.2020 14:02

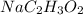 solution is 0.782 m
solution is 0.782 m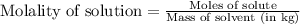 .....(1)
.....(1)