Chemistry, 28.06.2021 15:40 negativechill
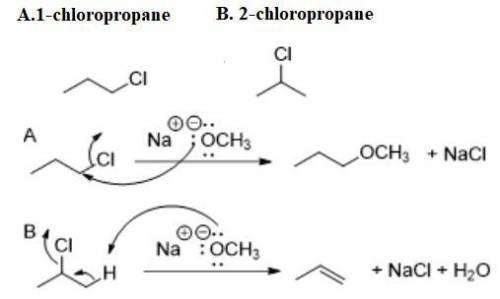
Compound A and compound B are constitutional isomers with molecular formula C3H7Cl. When compound A is treated with sodium methoxide, a substitution reaction predominates. When compound B is treated with sodium methoxide, an elimination reaction predominates.
Required:
Propose structures A and B.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 12:30
Given 7.65 g of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100% yield?
Answers: 3

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1
You know the right answer?
Compound A and compound B are constitutional isomers with molecular formula C3H7Cl. When compound A...
Questions





Mathematics, 24.04.2020 15:49




Chemistry, 24.04.2020 15:49





Mathematics, 24.04.2020 15:49




Mathematics, 24.04.2020 15:49

Social Studies, 24.04.2020 15:49