
Chemistry, 28.06.2021 19:40 yennifervilleda05
Pls help asap ! For each of these equations, predict the effects of temperature on the spontaneity of the reaction
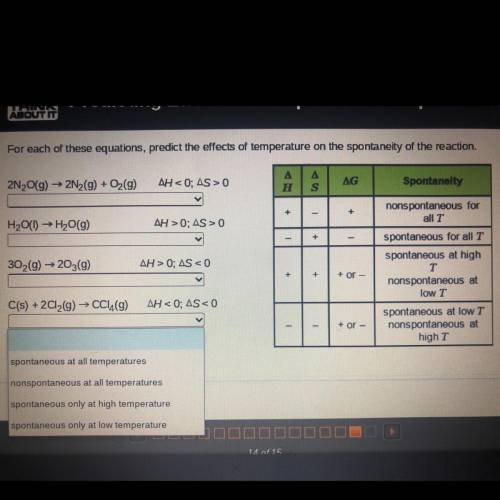

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 23.06.2019 08:00
What is the temperature in kelvin of a gas if it is allowed to expand from 1.50 l to 4.50 l? the initial temperature is 10.0°c and pressure is constant throughout the change. which equation should you use? t2= v2/v1 t1 what is the final temperature? ⇒ 849 k these are the answers.
Answers: 1


Chemistry, 23.06.2019 11:00
Suppose you increase your walking speed from 7 m/s to 15 m/s in a period of 1 s. what is your acceleration?
Answers: 1
You know the right answer?
Pls help asap ! For each of these equations, predict the effects of temperature on the spontaneity o...
Questions

English, 18.11.2020 06:10




Mathematics, 18.11.2020 06:10



Mathematics, 18.11.2020 06:10


Mathematics, 18.11.2020 06:10


Mathematics, 18.11.2020 06:10

Social Studies, 18.11.2020 06:10

Mathematics, 18.11.2020 06:10

Mathematics, 18.11.2020 06:10

English, 18.11.2020 06:10

English, 18.11.2020 06:10

History, 18.11.2020 06:10

Mathematics, 18.11.2020 06:10

Biology, 18.11.2020 06:10



