b) 15g NO

Chemistry, 29.06.2021 14:50 devin030505
Identify the substance which has same number of molecules in 14g N2
a) 3.4g NH3
b) 15g NO
c)64g O2
d) 32g SO2
e)1g H2
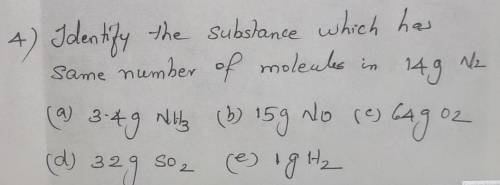

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1
You know the right answer?
Identify the substance which has same number of molecules in 14g N2
a) 3.4g NH3
b) 15g NO
b) 15g NO
Questions


Geography, 25.10.2021 06:40

Mathematics, 25.10.2021 06:40

Arts, 25.10.2021 06:40





Mathematics, 25.10.2021 06:40


History, 25.10.2021 06:40

Spanish, 25.10.2021 06:40

Mathematics, 25.10.2021 06:40

Mathematics, 25.10.2021 06:50

Social Studies, 25.10.2021 06:50



Arts, 25.10.2021 06:50

Biology, 25.10.2021 06:50

History, 25.10.2021 06:50



