
Chemistry, 29.06.2021 19:00 reagancunningham2004
The equilibrium constant (K p) for the interconversion of PCl 5 and PCl 3 is 0.0121:
PCl5 (g) → PCl3 (g) + Cl2 (g)
A vessel is charged with PCl 5 giving an initial pressure of 0.123 atm and yields PCl 3 and Cl 2. At equilibrium, the partial pressure of PCl 3 is atm.
A) 0.0782.
B) 0.0455.
C) 0.0908.
D) 0.0330.
E) 0.123.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
The equilibrium constant (K p) for the interconversion of PCl 5 and PCl 3 is 0.0121:
PCl5 (g) → PCl...
Questions

Biology, 25.09.2019 11:30


History, 25.09.2019 11:30

Health, 25.09.2019 11:30



History, 25.09.2019 11:30


Mathematics, 25.09.2019 11:30


Chemistry, 25.09.2019 11:30


Chemistry, 25.09.2019 11:30


English, 25.09.2019 11:30

Mathematics, 25.09.2019 11:30

Spanish, 25.09.2019 11:30

Mathematics, 25.09.2019 11:30

History, 25.09.2019 11:30

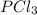 is 0.0330 atm.
is 0.0330 atm.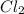 . Hence, let us assume that x quantity of
. Hence, let us assume that x quantity of 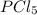 is decomposed and gives x quantity of
is decomposed and gives x quantity of 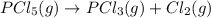
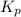 of this reaction is as follows.
of this reaction is as follows.![K_{p} = \frac{[PCl_{3}][Cl_{2}]}{[PCl_{5}]}\\0.0121 = \frac{x \times x}{(0.123 - x)}\\x = 0.0330](/tpl/images/1386/0346/b037d.png)


