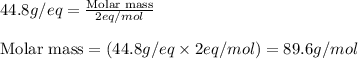Chemistry, 30.06.2021 05:50 CelesteN64
Calculate the molecular weight of a dibasic acid.0.56gm of which is required 250ml of N/20 sodium hydroxide solution for neutralization.

Answers: 3
Another question on Chemistry



Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2

Chemistry, 22.06.2019 23:30
To find the work done, the force exerted and distance moved are multiplied. a couch is moved twice before you are happy with its placement. the same force was used to move the couch both times. if more work is done the first time it is moved, what do you know about the distance it was moved? a) when more work was done, the couch was moved the same distance. b) when more work was done, the couch was moved less. c) when more work was done, the couch was moved further. d) when more work was done, the couch wasn't moved at all.
Answers: 1
You know the right answer?
Calculate the molecular weight of a dibasic acid.0.56gm of which is required 250ml of N/20 sodium hy...
Questions


World Languages, 19.05.2021 20:00

Mathematics, 19.05.2021 20:00

Social Studies, 19.05.2021 20:00

Mathematics, 19.05.2021 20:00


Chemistry, 19.05.2021 20:00


Chemistry, 19.05.2021 20:00

English, 19.05.2021 20:00




Chemistry, 19.05.2021 20:00

Mathematics, 19.05.2021 20:00

Mathematics, 19.05.2021 20:00

Mathematics, 19.05.2021 20:00

Mathematics, 19.05.2021 20:00


Mathematics, 19.05.2021 20:00

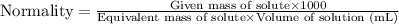 ....(1)
....(1)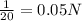
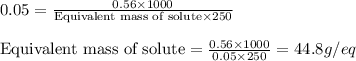
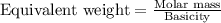 .....(2)
.....(2)