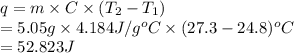Chemistry, 30.06.2021 19:30 sydneyclack
You heat a 5.05 g piece of titanium to 98.2 oC and place it into 20.00 mL of room temperature water (24.8 oC ). The temperature of the water rises to 27.3 oC. The specific heat of water is 4.184 J/goC. The density of water is 0.997 g/mL. A. How much heat is absorbed by the water (in units of J)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3

Chemistry, 23.06.2019 03:00
Analyze the reaction to determine whether the reaction is exothermic or endothermic. explain your reasoning.
Answers: 1

You know the right answer?
You heat a 5.05 g piece of titanium to 98.2 oC and place it into 20.00 mL of room temperature water...
Questions


Mathematics, 20.09.2019 07:00




History, 20.09.2019 07:00


Mathematics, 20.09.2019 07:00

English, 20.09.2019 07:00

Mathematics, 20.09.2019 07:00




Social Studies, 20.09.2019 07:00




History, 20.09.2019 07:00

World Languages, 20.09.2019 07:00

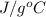
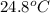
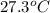
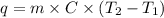
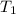 = initial temperature
= initial temperature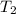 = final temperature
= final temperature