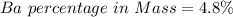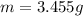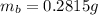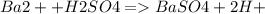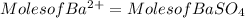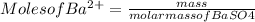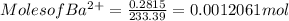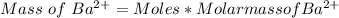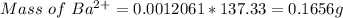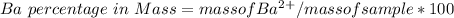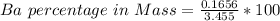Chemistry, 01.07.2021 04:20 mucciak2854
3. A 3.455-g sample of a mixture was analyzed for barium ion by adding a small excess of sulfuric acid to an aqueous solution of the sample. The resultant reaction produced a precipitate of barium sulfate, which was collected by filtration, washed, dried, and weighed. If 0.2815 g of barium sulfate was obtained, what was the mass percentage of barium in the sample

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 23.06.2019 04:31
Pls i will do pls imma diewhat forms white light? (4 points)a. combination of all wavelengths of ultraviolet light b. combination of all wavelengths of visible lightc. absorption of electromagnetic waves d. absorption of infrared rays
Answers: 2

Chemistry, 23.06.2019 08:30
If you had to research a particular disease or area of concern in veterinary medicine and science, which one would you choose? why?
Answers: 1

Chemistry, 23.06.2019 18:10
A1 forms when an acid is neutralized by a base. 1. salts can be neutral, or in solutions. salts of 2. strong acid–strong base reactions produce solutions with 3. water. salts formed from the neutralization of weak acids or weak 4. bases water. they produce solutions that are acidic or . basic. for example, the ph of a solution at the equivalence point is . greater than for a acid titration. solutions that resist changes in ph are called solutions. the buffer is the amount of acid or base that can be added to a buffer without changing the ph greatly.
Answers: 1
You know the right answer?
3. A 3.455-g sample of a mixture was analyzed for barium ion by adding a small excess of sulfuric ac...
Questions


Computers and Technology, 01.09.2020 17:01



Computers and Technology, 01.09.2020 17:01










Mathematics, 01.09.2020 17:01

Computers and Technology, 01.09.2020 17:01


Computers and Technology, 01.09.2020 17:01


Chemistry, 01.09.2020 17:01