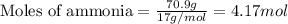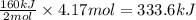2NH3(g)→N2(g)+3H2(g)

Chemistry, 01.07.2021 15:40 rowdycar313p0ao5k
A chemist measures the energy change
ΔH during the following reaction:
2NH3(g)→N2(g)+3H2(g)
ΔH=160kJUse the information to answer the following questions. This reaction is:.
a. endothermic
b. exothermic
Suppose 70.9 g of NH3 react. Will any heat be released or absorbed?
a. Yes, absorbed
b. Yes, released
c. No.
If you said heat will be released or absorbed in the second part of this question, calculate how much heat will be released or absorbed. Round your answer to 3 significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:30
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1
You know the right answer?
A chemist measures the energy change
ΔH during the following reaction:
2NH3(g)→N2(g)+3H2(g)
2NH3(g)→N2(g)+3H2(g)
Questions

Spanish, 23.04.2021 21:30



Mathematics, 23.04.2021 21:30



Spanish, 23.04.2021 21:30

History, 23.04.2021 21:30

Mathematics, 23.04.2021 21:30






Mathematics, 23.04.2021 21:30

History, 23.04.2021 21:30




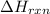 is positive for these reactions.
is positive for these reactions.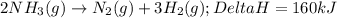
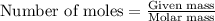 ......(1)
......(1)