Chemistry, 01.07.2021 16:00 ErrorNameTaken505
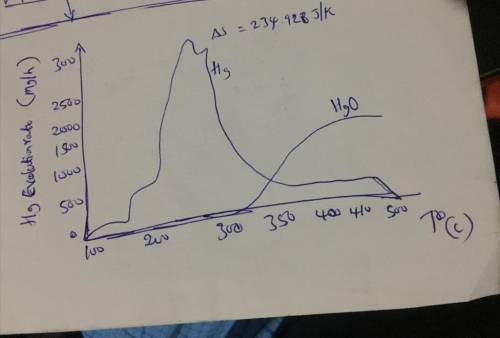
1. Show that heat flows spontaneously from high temperature to low temperature in any isolated system (hint: use entropy change that occurs during the process for your proof). 2. Work out the entropy change for the decomposition of mercuric oxide using mathematical and graphical arguments.

Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer.
Answers: 1


Chemistry, 23.06.2019 15:00
An isotope undergoes radioactive decay by emitting radiation that has no mass. what other characteristic does the radiation have?
Answers: 3
You know the right answer?
1. Show that heat flows spontaneously from high temperature to low temperature in any isolated syste...
Questions


Mathematics, 06.10.2019 04:30


Social Studies, 06.10.2019 04:30

English, 06.10.2019 04:30

Biology, 06.10.2019 04:30

Mathematics, 06.10.2019 04:30


Mathematics, 06.10.2019 04:30

Health, 06.10.2019 04:30

Social Studies, 06.10.2019 04:30

Social Studies, 06.10.2019 04:30

Mathematics, 06.10.2019 04:30



Business, 06.10.2019 04:30

Social Studies, 06.10.2019 04:30


Biology, 06.10.2019 04:30