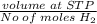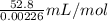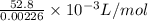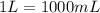Chemistry, 01.07.2021 16:30 izearaholland7308
Calculate the Experimental Molar Volume in L/mol of the Hydrogen gas, H2, if the volume of H2 at STP is 52.8 mL and the mass of Magnesium metal, Mg, used in the experiment is 0.055 g.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 23:00
If two identical atoms are bonded,what kind of molecule is formed
Answers: 1

Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3

Chemistry, 23.06.2019 02:30
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2

Chemistry, 23.06.2019 15:00
Solve this problem using the appropriate law. (remember that ) what is the pressure of 1.9 mols of nitrogen gas in a 9.45 l tank and at a temperature of 228 k?
Answers: 1
You know the right answer?
Calculate the Experimental Molar Volume in L/mol of the Hydrogen gas, H2, if the volume of H2 at STP...
Questions

Chemistry, 23.10.2019 03:00



Social Studies, 23.10.2019 03:00


Chemistry, 23.10.2019 03:00

Social Studies, 23.10.2019 03:00

History, 23.10.2019 03:00



History, 23.10.2019 03:00



Chemistry, 23.10.2019 03:00



Mathematics, 23.10.2019 03:00

Mathematics, 23.10.2019 03:00


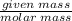
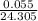 moles
moles