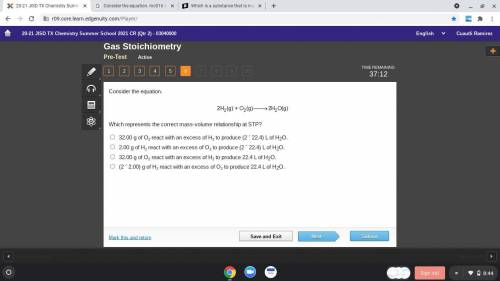
Consider the equation.
Which represents the correct mass-volume relationship at STP?
32.00 g...

Chemistry, 01.07.2021 17:00 juliannabartra
Consider the equation.
Which represents the correct mass-volume relationship at STP?
32.00 g of O2 react with an excess of H2 to produce (2 ´ 22.4) L of H2O.
2.00 g of H2 react with an excess of O2 to produce (2 ´ 22.4) L of H2O.
32.00 g of O2 react with an excess of H2 to produce 22.4 L of H2O.
(2 ´ 2.00) g of H2 react with an excess of O2 to produce 22.4 L of H2O.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1
You know the right answer?
Questions


Mathematics, 19.08.2019 13:50

History, 19.08.2019 13:50

Physics, 19.08.2019 13:50

Biology, 19.08.2019 13:50




History, 19.08.2019 13:50

Biology, 19.08.2019 13:50

Mathematics, 19.08.2019 13:50



Business, 19.08.2019 13:50

Mathematics, 19.08.2019 13:50

Mathematics, 19.08.2019 13:50



Health, 19.08.2019 13:50

Mathematics, 19.08.2019 13:50



