
Chemistry, 02.07.2021 04:30 montanolumpuy
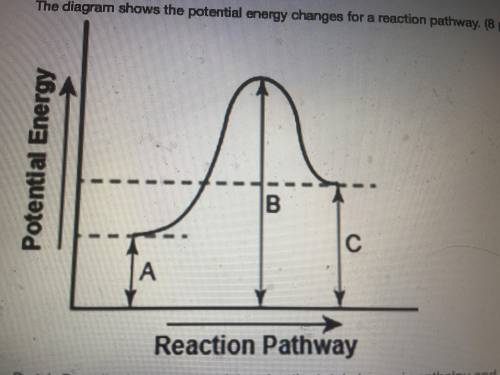
The diagram shows the potential energy changes for a reaction pathway.
Part 1: Describe how you can determine the total change in enthalpy and activation energy from the diagram, and if each is positive or negative.
Part 2: Describe how the curve will look if the reaction was exothermic. Be sure to mention changes in the potential energies of the reactants and products and the sign changes of the enthalpy.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1
You know the right answer?
The diagram shows the potential energy changes for a reaction pathway.
Part 1: Describe how you can...
Questions

Physics, 14.07.2019 20:30




Physics, 14.07.2019 20:30


History, 14.07.2019 20:30



History, 14.07.2019 20:30


Physics, 14.07.2019 20:30

History, 14.07.2019 20:30

Mathematics, 14.07.2019 20:30

History, 14.07.2019 20:30



Social Studies, 14.07.2019 20:30

Mathematics, 14.07.2019 20:30

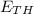 , B and the heat content of the reactants, A
, B and the heat content of the reactants, A

