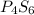Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Arock can be broken down into different kinds of substances by physical processes. no chemical reactions are needed to separate different parts of a rock into pure substances. this is because a rock is a(n)
Answers: 1

Chemistry, 21.06.2019 20:30
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

You know the right answer?
Compound X has a molar mass of 316.25 g*mol^-1 and the following composition:
element & mass %<...
Questions

English, 25.11.2020 09:10

Mathematics, 25.11.2020 09:10

History, 25.11.2020 09:10

Mathematics, 25.11.2020 09:10

History, 25.11.2020 09:10

Biology, 25.11.2020 09:10


Mathematics, 25.11.2020 09:20

Computers and Technology, 25.11.2020 09:20

Mathematics, 25.11.2020 09:20


Social Studies, 25.11.2020 09:20



Mathematics, 25.11.2020 09:20


Mathematics, 25.11.2020 09:20


Social Studies, 25.11.2020 09:20