

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
Decide if the following statements are true or false.
a. Ca has a larger ionization energy than Ca2...
Questions



Chemistry, 01.04.2020 03:10

Mathematics, 01.04.2020 03:10

History, 01.04.2020 03:10






Mathematics, 01.04.2020 03:10

Chemistry, 01.04.2020 03:10

Mathematics, 01.04.2020 03:10

Mathematics, 01.04.2020 03:10

Mathematics, 01.04.2020 03:10


English, 01.04.2020 03:10



Spanish, 01.04.2020 03:10

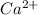 - False
- False
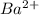 - False
- False
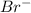 - True
- True
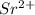 is larger than Sr - False
is larger than Sr - False

