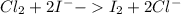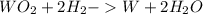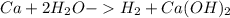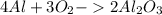Chemistry, 06.07.2021 22:10 alinadancer2717
Balance the following reactions and identify the species that have been oxidized and the species that have been reduced.
CL2 +I- > I2 +CL-
WO2 + H2 > W + H2O
CA + H2O > H2 + CA(OH)2
AL+ O2 > AL2O3

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Solar energy is energy from the sun that is converted into thermal or energy. a. nuclear b. mechanical c. electrical d. chemical
Answers: 2

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3
You know the right answer?
Balance the following reactions and identify the species that have been oxidized and the species tha...
Questions

Computers and Technology, 25.11.2019 19:31

Mathematics, 25.11.2019 19:31



Mathematics, 25.11.2019 19:31

History, 25.11.2019 19:31

Mathematics, 25.11.2019 19:31


History, 25.11.2019 19:31




Computers and Technology, 25.11.2019 19:31




Physics, 25.11.2019 19:31

Biology, 25.11.2019 19:31


Social Studies, 25.11.2019 19:31