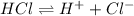Chemistry, 07.07.2021 01:00 laytonlutz
A Bronsted-Lowry acid is defined as a substance that . A Bronsted-Lowry acid is defined as a substance that . increases Ka when placed in H2O increases [OH-] when placed in H2O acts as a proton donor acts as a proton acceptor decreases [H ] when placed in H2O

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:10
How do forces between particles in gases compare to forces in the other states of matter? o a. the forces in gases are stronger than forces in solids but weaker than forces in liquids. o b. the forces in gases are weaker than forces in solids but stronger than forces in liquids. o c. the forces in gases are weaker than forces in solids and liquids. o d. the forces in gases are stronger than forces in solids and liquids. submit
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3
You know the right answer?
A Bronsted-Lowry acid is defined as a substance that . A Bronsted-Lowry acid is defined as a substan...
Questions

Chemistry, 14.01.2020 02:31

Mathematics, 14.01.2020 02:31

Mathematics, 14.01.2020 02:31

Biology, 14.01.2020 02:31






Mathematics, 14.01.2020 02:31

Mathematics, 14.01.2020 02:31


History, 14.01.2020 02:31


Biology, 14.01.2020 02:31


History, 14.01.2020 02:31

Mathematics, 14.01.2020 02:31