Chemistry, 08.07.2021 01:50 briannmalcolmp3l4pz
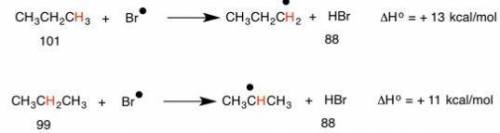
Which of the following statements correctly explains why bromination reactions are more selective than chlorination reactions.
a. bromine radical is less stable than chlorine radical, so it is more reactive and less choosy
b. bromine radical is more stable than chlorine radical, so it is more reactive and less choosy
c. bromine radical is more stable than chlorine radical, so it is less reactive and more choosy
d. bromine radical is less stable than chlorine radical, so it is less reactive and more choosy
e. relative radical stability is 3' radicals > 2" radicals> 1 radicals when bromine radicals snatch hydrogens from alkanes, but when chlorine radicals snatch hydrogens the resulting alkyl radical stability is 3 radicals < 2 radicals< 1' radicals

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1
You know the right answer?
Which of the following statements correctly explains why bromination reactions are more selective th...
Questions

Chemistry, 04.05.2021 06:50



Mathematics, 04.05.2021 06:50

Mathematics, 04.05.2021 06:50

Mathematics, 04.05.2021 06:50



Computers and Technology, 04.05.2021 06:50

Mathematics, 04.05.2021 06:50

Chemistry, 04.05.2021 06:50

Mathematics, 04.05.2021 06:50

Mathematics, 04.05.2021 06:50

Biology, 04.05.2021 06:50

Mathematics, 04.05.2021 06:50



Mathematics, 04.05.2021 06:50


History, 04.05.2021 06:50