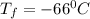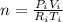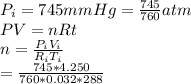A gas sample has a volume of 4250 mL at 15 degree Celsius and 745 mmHg. What is the final temperature, in degree Celsius, after the sample is transferred to a different container with a volume of 2.50 L and a pressure of 1.20 atm if the amount of gas does not change?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1
You know the right answer?
A gas sample has a volume of 4250 mL at 15 degree Celsius and 745 mmHg. What is the final temperatur...
Questions

Mathematics, 26.09.2021 18:10

Mathematics, 26.09.2021 18:10



History, 26.09.2021 18:10

Mathematics, 26.09.2021 18:10

History, 26.09.2021 18:10

Mathematics, 26.09.2021 18:10


Business, 26.09.2021 18:10

English, 26.09.2021 18:10

Mathematics, 26.09.2021 18:10

History, 26.09.2021 18:10



Mathematics, 26.09.2021 18:10


Biology, 26.09.2021 18:10

Mathematics, 26.09.2021 18:10

Mathematics, 26.09.2021 18:10