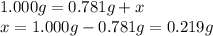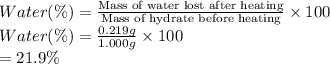This question has multiple parts.
Part A:
A sample of unknown hydrate, AC-XH20, has a mass of...

Chemistry, 08.07.2021 18:40 brianmondesir1owahud
This question has multiple parts.
Part A:
A sample of unknown hydrate, AC-XH20, has a mass of 1.000 g before heating and a
mass of 0.781 g after heating. What is the experimental percentage of water in this
unknown hydrate?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 05:20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1
You know the right answer?
Questions



Computers and Technology, 31.05.2020 03:02


Chemistry, 31.05.2020 03:02

Mathematics, 31.05.2020 03:02

Mathematics, 31.05.2020 03:02


Mathematics, 31.05.2020 03:03

Mathematics, 31.05.2020 03:03


Mathematics, 31.05.2020 03:03