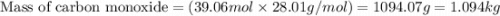Chemistry, 08.07.2021 18:50 leilaford2003
What mass of carbon monoxide is needed to react with 2.08 kg of iron oxide? > Fe^2O^3 + 3CO --> 2Fe + 3CO^2
20 points, plz help and plz show how you work
I WILL MARK YOU BRAINIEST IF U ANSWER WITH FULL WORKING OUT!

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1
You know the right answer?
What mass of carbon monoxide is needed to react with 2.08 kg of iron oxide? > Fe^2O^3 + 3CO -->...
Questions


Mathematics, 01.09.2019 01:50



History, 01.09.2019 01:50


Business, 01.09.2019 01:50




Mathematics, 01.09.2019 01:50




Geography, 01.09.2019 01:50

Arts, 01.09.2019 01:50

History, 01.09.2019 01:50



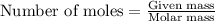 ......(1)
......(1)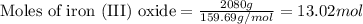
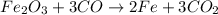
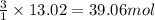 of CO
of CO