
An element M reacts with another element X to form 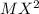 . In terms of loss or gain of electron, identify the element oxidized and reduced.
I need help in understanding this question, I know what a redox reaction is in terms of loss or gain of electron. However, I am not able to solve this question. I think that's maybe because the oxidation state or valency is not given for the unknown elements. Like, how do I know if the element is gaining or loosing electron when I don't know it's valency.
. In terms of loss or gain of electron, identify the element oxidized and reduced.
I need help in understanding this question, I know what a redox reaction is in terms of loss or gain of electron. However, I am not able to solve this question. I think that's maybe because the oxidation state or valency is not given for the unknown elements. Like, how do I know if the element is gaining or loosing electron when I don't know it's valency.
I repeat, I need to UNDERSTAND this question, I already know the answer but am just confused about how to solve it...

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:30
One mole of zinc has a mass of 65.4 grams. approximately how many atoms of zinc are present in one mole of zinc? 32 × 1023 atoms 6 × 1023 atoms 66 atoms 65 atoms
Answers: 1


Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2
You know the right answer?
An element M reacts with another element X to form . In terms of loss or gain of electron, identify...
Questions





Social Studies, 01.10.2019 00:00

English, 01.10.2019 00:00

Mathematics, 01.10.2019 00:00



History, 01.10.2019 00:00


English, 01.10.2019 00:00

Computers and Technology, 01.10.2019 00:00

History, 01.10.2019 00:00

Health, 01.10.2019 00:00



History, 01.10.2019 00:00


English, 01.10.2019 00:00



