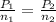Chemistry, 09.07.2021 17:30 misrachel03
A gas bottle contains 0.650 mol of gas at 730. mmHg pressure. If the final pressure is 1.15 atm, how many moles of gas were added to the bottle

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

You know the right answer?
A gas bottle contains 0.650 mol of gas at 730. mmHg pressure. If the final pressure is 1.15 atm, how...
Questions


English, 05.11.2019 05:31




Mathematics, 05.11.2019 05:31

English, 05.11.2019 05:31

Social Studies, 05.11.2019 05:31

English, 05.11.2019 05:31

English, 05.11.2019 05:31

Geography, 05.11.2019 05:31









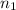 = 0.650 mol,
= 0.650 mol, 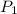 = 730 mm Hg (1 mm Hg = 0.00131579 atm) = 0.96 atm
= 730 mm Hg (1 mm Hg = 0.00131579 atm) = 0.96 atm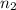 = ?,
= ?, 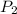 = 1.15 atm
= 1.15 atm