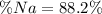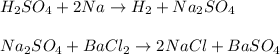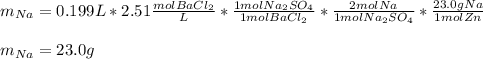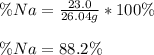Chemistry, 10.07.2021 02:00 jetblackcap
A 26.04 g mixture of zinc and sodium is reacted with a stoichiometric amount of sulfuric acid. The reaction mixture is then reacted with 199 mL of 2.51 M barium chloride to produce the maximum possible amount of barium sulfate. Determine the percent sodium by mass in the original mixture.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Water is important for the of cells. a: size, shape, and temperature b: temperature, color, and odor c: color, odor, and size d: shape, temperature, and color
Answers: 2

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

You know the right answer?
A 26.04 g mixture of zinc and sodium is reacted with a stoichiometric amount of sulfuric acid. The r...
Questions


Mathematics, 29.08.2019 20:30

Biology, 29.08.2019 20:30



Computers and Technology, 29.08.2019 20:30


History, 29.08.2019 20:30




Social Studies, 29.08.2019 20:30



Mathematics, 29.08.2019 20:30

Mathematics, 29.08.2019 20:30