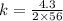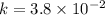Chemistry, 10.07.2021 14:00 megankbrown
For a reaction: aA → Products, [A]o -4.3 M, and the first two half-lives are 56 and 28 minutes, respectively. Calculate k (without units). Show Work!
A. 7.7 x 10^-2
B. 4.2 x 10^-3
C. 3.8 x 10^-2
D. 8.3 x 10^-3
E. None of these

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2


Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1

You know the right answer?
For a reaction: aA → Products, [A]o -4.3 M, and the first two half-lives are 56 and 28 minutes, resp...
Questions

English, 18.04.2021 21:00


English, 18.04.2021 21:00


Mathematics, 18.04.2021 21:00




Biology, 18.04.2021 21:00

Mathematics, 18.04.2021 21:00

Computers and Technology, 18.04.2021 21:00


Mathematics, 18.04.2021 21:00

Mathematics, 18.04.2021 21:00

Computers and Technology, 18.04.2021 21:00

English, 18.04.2021 21:00

Mathematics, 18.04.2021 21:00



Arts, 18.04.2021 21:00

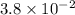
![[A]_o=4.3 M](/tpl/images/1392/0930/2ab48.png)
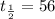 minutes
minutes minutes
minutes![t_{\frac{1}{2}}=\frac{[A]_o}{2k}](/tpl/images/1392/0930/a6be7.png)