
Chemistry, 12.07.2021 01:00 franstirlacci
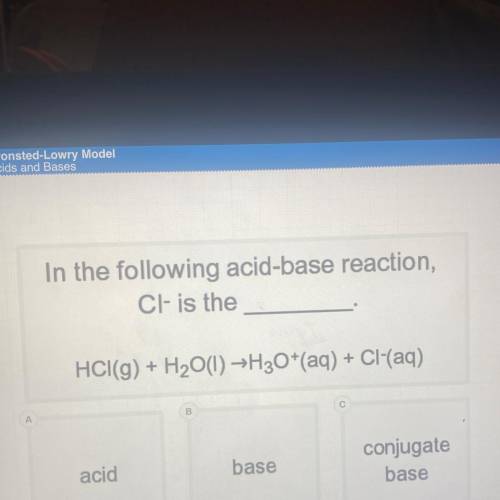
In the following acid-base reaction,
Cl- is the
HCI(g) + H2O(l) →H30+(aq) + Cl(aq)
acid
base
conjugate
base

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1
You know the right answer?
In the following acid-base reaction,
Cl- is the
HCI(g) + H2O(l) →H30+(aq) + Cl(aq)
acid...
HCI(g) + H2O(l) →H30+(aq) + Cl(aq)
acid...
Questions


Mathematics, 17.05.2021 17:50

Mathematics, 17.05.2021 17:50

Mathematics, 17.05.2021 17:50

English, 17.05.2021 17:50



English, 17.05.2021 17:50

Mathematics, 17.05.2021 17:50




English, 17.05.2021 17:50

Mathematics, 17.05.2021 17:50

Mathematics, 17.05.2021 17:50

Social Studies, 17.05.2021 17:50

Mathematics, 17.05.2021 17:50

Mathematics, 17.05.2021 17:50




