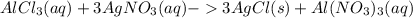Chemistry, 12.07.2021 19:30 swaggyd6603
If 13.50 mL of an aluminum chloride solution is needed to reach the equivalence point with 10.00 mL of 0.109 M silver nitrate solution, determine the molarity of the aluminum chloride solution.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2

Chemistry, 23.06.2019 02:50
For questions 1 and 2, consider the following experimental data.hydrogen emission lines were detected at the following wavelengths (in nm): 121.6102.697.395.093.8question 1use the electromagnetic radiation classifications below and figure 1-1 in the introductory information for this lab (in the lab manual) to determine the nf value for the experimental data provided? wavelength, ? (nm) 650 700 550 600 400 450 500 visible spectrum wavelength, ? (m) 11 10 3 10 10 10 8 10 5 10 10 -10 10 9 10 10 10 10 -12 10 microwave radio infrared x-ray ultraviolet gamma 1020 1019 1018 1 1016 015 1014 01 12 109108 frequency, v (hz)a.1b. 2c. 3d. 4e. 5question 2using the data for the emission line with the longest wavelength, the known value of nf (from question 1 in this prelab), and the value of ni (deduced from the ? and nf values) calculate the rydberg constant for hydrogen (rh) in units of m-1.a) 1.097 x 10-11 m-1b) 5.921 x 107 m-1c) 1.097 x 10-2 m-1d) 9.252 x 106 m-1e) 1.097 x 107 m-1
Answers: 3

Chemistry, 23.06.2019 10:40
Aliquid solution can be made select all that apply. dissolving solids into liquids, mixing liquids, dissolving gas solutes into liquids , mixing gases, mixing solids
Answers: 3

Chemistry, 23.06.2019 16:30
Ashell can hold a maximum of 32 electrons, what is the value of n?
Answers: 3
You know the right answer?
If 13.50 mL of an aluminum chloride solution is needed to reach the equivalence point with 10.00 mL...
Questions



Mathematics, 25.03.2021 03:40

English, 25.03.2021 03:40

Chemistry, 25.03.2021 03:40

Mathematics, 25.03.2021 03:40

English, 25.03.2021 03:40


Mathematics, 25.03.2021 03:40


Advanced Placement (AP), 25.03.2021 03:40

Mathematics, 25.03.2021 03:40

Health, 25.03.2021 03:40


Mathematics, 25.03.2021 03:40

History, 25.03.2021 03:40

Mathematics, 25.03.2021 03:40

Mathematics, 25.03.2021 03:40

History, 25.03.2021 03:40