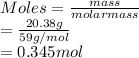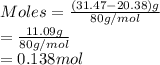Chemistry, 12.07.2021 19:40 Annabel9554
A 20.38 gram sample of cobalt is heated in the presence of excess sulfur. A metal sulfide is formed with a mass of 31.47 g. Determine the empirical formula of the metal sulfide.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3


Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 23.06.2019 05:30
What is the morality of 2.50 l of solution that contains 1.85 mol of anhydrous sodium tetraborate?
Answers: 1
You know the right answer?
A 20.38 gram sample of cobalt is heated in the presence of excess sulfur. A metal sulfide is formed...
Questions


English, 16.05.2020 20:57



Mathematics, 16.05.2020 20:57


Computers and Technology, 16.05.2020 20:57


Chemistry, 16.05.2020 20:57


Mathematics, 16.05.2020 20:57



History, 16.05.2020 20:57

Mathematics, 16.05.2020 20:57

Mathematics, 16.05.2020 20:57

Chemistry, 16.05.2020 20:57


Health, 16.05.2020 20:57

 .
.